Cambridge University Hospitals NHS Foundation Trust (CUH) has become the first hospital in Europe to deliver histotripsy treatment to a patient outside of a clinical trial, after being fast-tracked by the government - marking a major milestone in NHS cancer care.
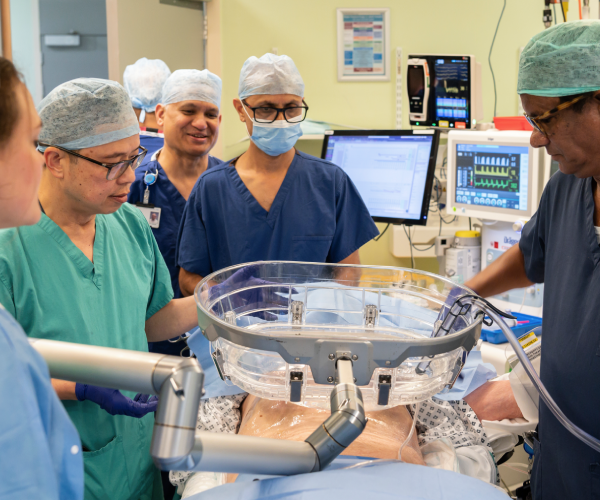
The pioneering procedure which uses ultrasound energy to target and destroy tumours took place at Addenbrooke’s Hospital, where Roger Jackson from Bedford underwent the incisionless treatment for liver cancer.
Understanding how we can treat cancer more precisely through kinder non-invasive methods will be at the forefront of care in our planned Cambridge Cancer Research Hospital, which will bring clinical and research expertise together into one NHS building.
Histotripsy uses focused sound waves to generate microscopic “bubble clouds” from naturally occurring gases present in targeted tumour tissues. The bubbles form and collapse in microseconds, creating mechanical forces that destroy cancer cells without the need for surgery, radiation, or chemotherapy. With treatment taking as little as 30 minutes and usually with minimal or no pain, patients can recover quickly and spend less time in hospital, with treatment performed as a day case.
Watch the first histotripsy procedure at CUH
Link: https://youtu.be/gAVwrlnfVt0
Dr Teik Choon See, consultant interventional radiologist at CUH, led the procedure.
"Histotripsy represents a major and exciting step forward in cancer treatment. It allows us to target tumours more precisely while sparing surrounding healthy tissue, offering patients a safer and faster alternative to traditional therapies."
Dr Teik Choon See

Dr See added: “What is even more promising is in some reported cases, after the sound waves break apart the tumour, the patient’s immune response may become activated and clear up some remaining cancerous tissues, showing real hope for patients.”

Roger Jackson, 80, said: “I feel privileged to be the first NHS patient and to receive this care was an amazing experience. It is impressive to think that sound waves can treat cancer, without the need for patients like me to go through intensive surgery, at what already is a stressful time. I’m hugely grateful to the team at Addenbrooke’s for their specialist care and expertise.”
After treatment last week, Mr Jackson was discharged the following day and is back at home. He said he is now looking forward to spending time with his family, including his sons, grandchildren and great-grandchildren.

The installation of the Edison Histotripsy System at Addenbrooke’s was made possible by a generous donation to the University of Cambridge from the Li Ka Shing Foundation, a long-standing supporter of cancer research in Cambridge. The technology, developed by US-based HistoSonics, has already treated over 2,000 patients worldwide following the Food and Drug Administration (FDA) clearance for the destruction of liver tumours in 2023.
Roger Jackson’s treatment is the first histotripsy procedure to take place after the equipment was granted Unmet Clinical Need Authorisation (UCNA) in Great Britain enabling time-limited, controlled early access to the Histotripsy device under the UK’s Innovative Devices Access Pathway (IDAP) pilot programme.
Overseen by the Medicines and Healthcare products Regulatory Agency (MHRA), the UCNA enables early market access to the medical device under certain conditions prior to full regulatory approval, meaning NHS patients can benefit from technology years earlier than planned.
With preliminary funding from Addenbrooke’s Charitable Trust (ACT), treatment is initially being offered to selected patients with tumours from primary and secondary liver cancers. The National Institute for Health and Care Research (NIHR) is exploring initiatives to fund research into the clinical and cost-effectiveness of histotripsy. Further studies are underway to explore its use in other cancer types.
"This marks the beginning of a new generation in cancer treatment. We are lighting the fuse beneath the technological revolution, transforming care for NHS patients."
Health and Social Care Secretary Wes Streeting
Health and Social Care Secretary Wes Streeting said:
“By slashing red tape, we’ve made sure this game-changing new cancer treatment has reached the NHS front line quicker, and I'm proud to say British patients are now the first in Europe to benefit.
“This government has streamlined approval processes to create an NHS fit for the future - protecting patients while unleashing the full potential of our scientists and NHS staff so they can deliver world-class care.”
"Histotripsy represents a hugely exciting and new era of cancer innovation and care. With faster recovery times and shorter hospital stays, this not only reduces the strain on our hospital beds, but it also frees up surgeons to focus on the more complex cancer cases, helping to cut waiting times."
Roland Sinker, chief executive of CUH

Roland Sinker, CEO of CUH said: “We are delighted to be at the forefront of this new ground-breaking technology and understanding how we can treat cancer more accurately and precisely, a position we aim to strengthen further with our planned Cambridge Cancer Research Hospital.”
The specialist hospital, set to be built on Europe’s largest life science campus, the Cambridge Biomedical Campus, is a partnership between Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge. By bringing world-leading scientists and clinical expertise together in one NHS building, the new hospital will treat patients across the East of England and will accelerate research and innovations to change the story of cancer across the UK and beyond. Find out more here (opens in a new tab).
Lawrence Tallon, Chief Executive of the MHRA, said: “This milestone shows how smart, agile regulation can help bring promising new treatments to patients sooner. Through the Innovative Devices Access Pathway, we at the MHRA have worked with partners across the health system to safely make early access to this technology possible.
“My congratulations to the team at Cambridge University Hospitals on this breakthrough – their work demonstrates how collaboration can unlock innovation for patients and deliver faster access to care.”
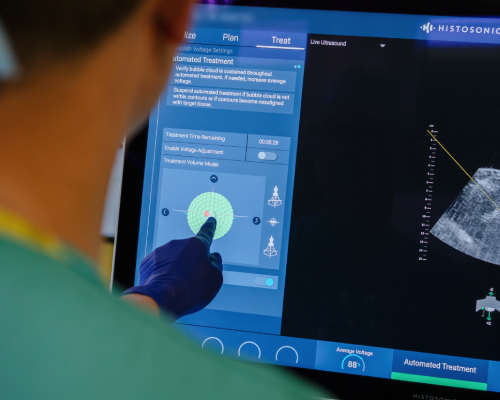
Treatment for Histotripsy
Addenbrooke’s is currently setting up a referral pathway, so the histotripsy technology can be made available to patients at Addenbrooke’s and beyond. External referrals will be considered through a consultant referral, and suitability for the treatment will be decided by medical teams based on the cancer location, size, extent and overall patient’s fitness.
No other provider is offering histotripsy in the UK at the moment.
We recommend patients speak to their consultant if they have any questions about being referred for treatment. If you already have a referral, and have further questions for the Cambridge team, please email cuh.histotripsyenquiries@nhs.net.