Featured news
All news

Professor Rebecca Fitzgerald elected as Fellow of AACR
The Research Lead for Cambridge Cancer Research Hospital, Professor Rebecca Fitzgerald, is elected as a 2026 Fellow of the American Association for Cancer Research.

Hot flush treatment has anti-breast cancer activity, study finds
A drug mimicking the hormone progesterone has anti-cancer activity when used together with conventional anti-oestrogen treatment for women with breast cancer, a new Cambridge-led trial has found.

Behind the scenes: Stand Up To Cancer live from Addenbrooke’s
Last week a special programme for Stand Up To Cancer presented by Davina McCall was broadcast live from Addenbrooke’s Hospital.

Collaboration with new Digital Health and Surgical Training Centre
Carrie Symington, Senior Improvement Manager, and Elaine Chapman, Lead Nurse for the Cambridge Cancer Research Hospital (CCRH) project recently had the pleasure of visiting the Cambridge Surgical Training Centre (CSTC) on the Quorum site. They discuss what the new area will offer staff across the region, what they learned from the tour, and the opportunities the Centre presents for staff working in the future Cancer hospital.

Pioneering trial offers hope on world pancreatic cancer day
A pioneering trial could improve treatment for late-stage pancreatic cancer by reducing side effects from chemotherapy.

Addenbrooke’s to host live Channel 4 programme for Stand Up To Cancer
A pioneering Channel 4 programme presented by Davina McCall will be broadcast live from Addenbrooke’s Hospital as part of this year’s Stand Up To Cancer 2025. It will highlight the expertise, innovation and dedication of clinicians in Cambridge, as 'Cancer Clinic: Live' broadcasts from a special one-off cancer clinic at Addenbrooke’s on the evening of Friday 12th December.
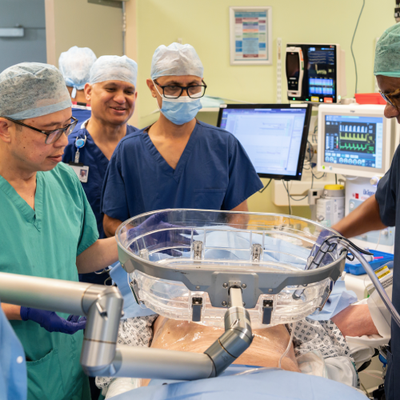
Addenbrooke’s begins innovative liver cancer treatment for first NHS patients in Europe
Cambridge University Hospitals NHS Foundation Trust (CUH) has become the first hospital in Europe to deliver histotripsy treatment to a patient outside of a clinical trial, after being fast-tracked by the government - marking a major milestone in NHS cancer care.

Pioneering one-stop kidney clinic provides same day diagnosis and halves cancer waiting times
In a UK first, a one-stop kidney cancer clinic at Addenbrooke’s Hospital is pioneering an ultra-fast way of diagnosing cancer, which is cutting waiting times for patients by a month.

Trust clinicians to feature in Cambridge Film Festival
Shades of Survival will be shown on 26 & 28 October at the Cambridge Film Festival, exploring inequalities in breast cancer care for Black women.

Cambridge Dragon Boat Festival raises vital funds for Cambridge Cancer Research Hospital
Teams taking part in this year’s 2025 Cambridge Dragon Boat Festival have beat last year’s fundraising total to raise an impressive £40,000 for the new Cambridge Cancer Hospital.

Pioneering breast cancer programme opens in more hospitals across the East
Patients who join the Personalised Breast Cancer Programme, which was pioneered at Addenbrooke's Hospital in Cambridge, have their DNA read like a barcode, with the whole genome of their tumour sequenced and the results returned to inform treatment.

Social media star GK Barry visits Teenage Cancer Trust unit at CUH
Social media influencer and TV star Grace Keeling, known as GK Barry, visited Cambridge University Hospital’s NHS Foundation Trust (CUH) to spread some cheer to teenagers from the East of England region being treated for cancer.

Faster, more accessible clinical trial initiative being piloted in pancreatic cancer
The PemOla precision medicine trial in pancreatic cancer is exploring a new way of running clinical trials that can help more patients to get treatment closer to home.

Low-risk patients could avoid more aggressive breast cancer treatments, study shows
Targeted breast radiotherapy is as safe and effective as less focused approaches that have more side effects

Archaeological dig underway ahead of construction of Cambridge Cancer Research Hospital
The archaeological dig is underway on the site of the future Cambridge Cancer Research Hospital - a ground-breaking new hospital that will change the story of cancer forever.

'Pill-on-a-thread' could replace endoscopies for half of patients being monitored for oesophageal cancer
Capsule sponge tests could replace endoscopies for up to half of people being monitored for oesophageal cancer.

Cambridge to offer cutting-edge ultrasound treatment for NHS cancer patients in UK first
NHS patients at Addenbrooke’s Hospital will become the first in the UK and Europe to undergo incisionless ultrasound surgery using a cutting-edge ‘histotripsy machine’ as part of their cancer care.

CUH to benefit from new cutting-edge radiotherapy machine to speed up cancer treatment
Cambridge University Hospitals NHS Foundation Trust (CUH) is set to benefit from a new radiotherapy machine that will help cancer patients receive faster treatment.

Enhanced breast cancer screening in the UK could detect an extra 3,500 cancers per year, trial shows
Enhanced screening could detect 3,500 more breast cancers and save around 700 lives per year.

New approach to treating aggressive breast cancers shows significant improvement in survival
A new treatment approach significantly improves survival rates for patients with aggressive, inherited breast cancers, according to Cambridge researchers.





